Critical Recall of HeartSine AEDs:
Immediate Action Required to Prevent Potentially Fatal Device Failures

Last Updated
August 19, 2025
HeartSine AED Recall
August 15, 2025 — A recall has been issued for certain models of the Stryker HeartSine Automated External Defibrillator, a device used to treat victims of sudden cardiac arrest. The FDA reports over 190,000 units affected by this Class II recall, with devices possibly failing to deliver therapy during an emergency due to a critical circuit board defect. This defect can cause the AED to become completely inoperable at any point during its use, including while preparing to deliver a shock, during the shock itself, or immediately after. A failed AED in a cardiac emergency is not just a malfunction, it’s a missed chance to save a life.
Affected HeartSine AED Devices Include:
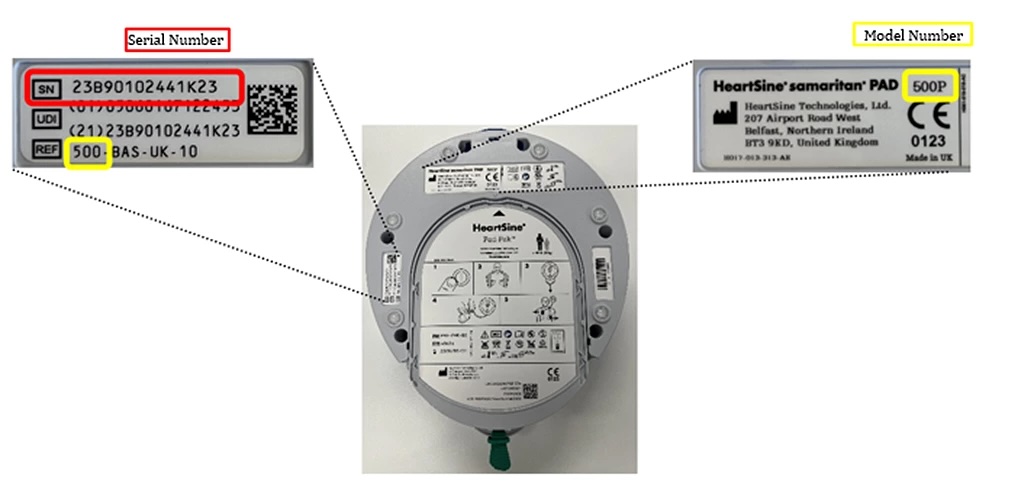
- HeartSine Samaritan PAD Models: 350P, 360P, 450P, 500P
- Serial numbers beginning with: 21, 22, 23, or 24, followed by B, D, E, G, or H
- Check your AED’s serial number to determine if it is part of the recall.
- Submit your device for destruction and replacement.
What You Must Do Immediately:
Should you replace your HeartSine AED?
While Stryker has suggested keeping affected AEDs in service if no alternative is available (claiming a “low probability” of failure) this advice significantly understates the risk. A cardiac arrest is an unpredictable, high-stakes emergency. A non-functional AED offers zero protection and can result in preventable death. We strongly urge all organizations, institutions, and individuals to replace affected devices with a reliable, rescue-ready alternative as soon as possible.

Rebates are available: [Note: Limited time]
Several trusted AED manufacturers are currently offering rebates and trade-in programs for organizations turning in recalled HeartSine Samaritan PADs. This is a great opportunity to upgrade to a reliable, FDA-cleared AED from a company with a strong safety record (often at a reduced cost).
These programs not only help with replacement costs but also ensure your location is protected with a functioning, dependable device. This Class II recall marks Stryker’s sixth AED recall in the past five years.
Many customers, needing a reliable, rescue-ready AED at all times, are switching to trusted, reliable AEDs. For organizations with multiple devices, or for help identifying a rescue-ready AED alternative, reach out to our team at MME for an option that’s right for your organization.customerservice@mmemed.com