Blog
Is your AED Rescue-ready and FDA Compliant?

An Automatic External Defibrillator, or AED, is something that most people tend to keep in their peripheral. AEDs just aren’t that important to most people… until something goes wrong. AEDs are designed to shock a heart’s rhythm when someone suffers a Sudden Cardiac Arrest (SCA). It’s impossible to predict when or where these emergencies will happen and there is a very short window to rescue the victim. When the clock starts, everyone starts looking for these cabinets, but sometimes there are none to be found. Sudden Cardiac Arrests can happen to anyone, anywhere, so how prepared would you be when facing this situation?
In a study of AED proximity and deployment time¹, researchers analyzed 78 sites with AEDs. In buildings with AEDs on the ground floor, most SCA emergencies happened less than 45ft from the AED. Total time to deploy the AEDs ranged from 50 seconds to two and a half minutes. Deployment time depends on a variety of factors including distance from emergency, accessibility of AED (sometimes, units are locked and only accessible by officer, staff, etc.), discussion time, visibility, and so on.
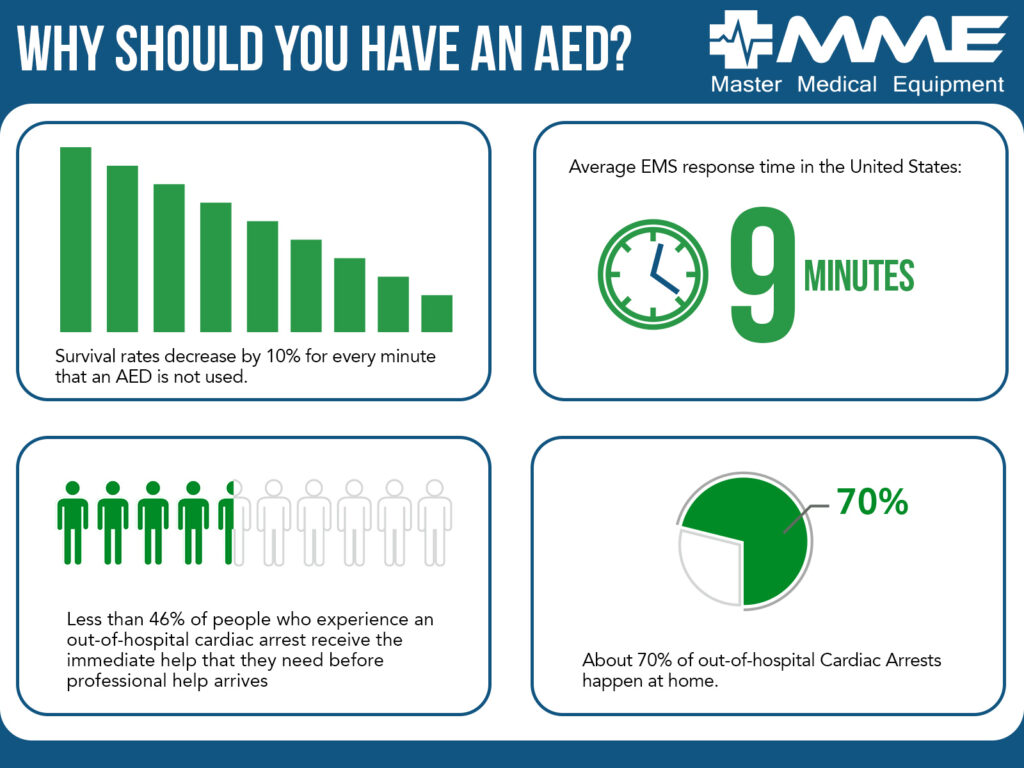
When facing a Sudden Cardiac Arrest, time is precious, and seconds can make the difference whether or not you can save a life. Expected survival rates decrease by a stunning 10% for every minute that goes by without application of an AED. On average, it takes first responders 7-14 minutes to get on scene, but permanent brain damage can occur in as little as four minutes. On-site, centrally located, and easy-to-find AEDs can make all the difference.
Another issue that poses itself is the FDA’s PMA guidelines for a range of AED’s that will be “End of Life.” This guideline means that a unit is non-FDA approved if the manufacturer did not file for a premarket approval application (PMA) by the revised deadline of February 3, 2022. No entity can buy or sell accessories for non-FDA-approved AEDs in the United States after February 3, 2022, effectively  killing non-approved AEDs and defibrillators. This means you will no longer be able to buy batteries and pads for FDA non-PMA units, and expired pads and batteries are unreliable when making a save.
killing non-approved AEDs and defibrillators. This means you will no longer be able to buy batteries and pads for FDA non-PMA units, and expired pads and batteries are unreliable when making a save.
Of course, certain places need to have AEDs, such as schools, markets, and office buildings, but we know a life-saving AED should be available and easy to locate everywhere. An example of AED placement that you might not expect is dentist offices. With potential complications due to sedation, the dental industry began to recommend AEDs in dental office, and even requires them in certain states:
Florida
Georgia
Illinois
Tennessee
Washington
New York
Texas
Mississippi
Massachusetts
Many older AEDs will be out of PMA approval after Feb 3, 2022, and will no longer be rescue-ready once their current pads and batteries pass their recommended shelf life. This is a growing risk for AEDs placed in schools, offices, and public places.
But what about your office? Your place of business? How can you protect people from the peripherals? Purchasing an AED and keeping it maintained is an assurance that you will be ready for cardiac arrest emergencies whenever it happens. There are many AEDs, so here is a list of ones that will be affected by the PMA deadline:
- Welch Allyn AED 10
- Cardiac Science 9200
- Heartsine 300P
- Philips Forerunner
- Physio-Control Lifepak 20
- Physio-Control Lifepak 500
- Philips Heartstart XL
- Philips Heartstart MRx
- Zoll M Series Defibrillator
- Zoll E Series Defibrillator
Now that we know what devices are going out, what devices are staying in? Any AED not on the above list will have parts and accessories ready for servicing to keep them ready for whenever you need them.
If you have an AED that is losing PMA approval, learn more about your options for remaining in compliance here. Not sure what type of AED would be best for you? Our friendly customer service is here to help you with whatever decisions you need. (866-468-9558) Purchasing an AED is assurance for both you and those around you, and MME is here to guide you into an affordable solution!
———————-
¹(https://pubmed.ncbi.nlm.nih.gov/29168550/) Full Reference: Telec W, Baszko A, Dąbrowski M, Dąbrowska A, Sip M, Puslecki M, Kłosiewicz T, Potyrała P, Jurczyk W, Maciejewski A, Zalewski R, Witt M, Ladny JR, Szarpak L. Automated external defibrillator use in public places: a study of acquisition time. Kardiol Pol. 2018;76(1):181-185. DOI: 10.5603/KP.a2017.0199. Epub 2017 Nov 23. PMID: 29168550.

Jacob Beals,
Writer, Master Medical Equipment
